2023 Volume 48 Issue 3 Pages 109-119
2023 Volume 48 Issue 3 Pages 109-119
Bisphenol A (BPA), one of the main components of industrial products, is clinically associated with the increased male infertility rate. However, the underlying molecular mechanism of the BPA-resulted reproductive toxicity is not fully elucidated. Voltage-dependent anion channel 1 (VDAC1) is a pore protein and located at the outer mitochondrial membrane. As a mitochondrial gatekeeper, VDAC1 controls the release of reactive oxygen species (ROS) and the metabolic and energetic functions of mitochondria, and serves as a critical player in mitochondrial-mediated apoptosis. Herein, we explored the role of VDAC1 in BPA-induced apoptosis of spermatogonia. The results showed that BPA increased spermatogonia cell line GC-1 spg cell apoptosis and intracellular ROS level, and suppressed AMPK/mTOR signaling pathway at a dose of 80 μM for 48 hr. Lentivirus-mediated short hairpin RNA targeting VDAC1 (Lv-shVDAC1) silenced VDAC1 expression and enhanced BPA-restricted cell viability. Knockdown of VDAC1 inhibited the apoptosis of BPA-treated GC-1 spg cells determined by with changes of the expressions of pro-apoptotic and anti-apoptotic proteins. Knockdown of VDAC1 also alleviated the BPA-triggered intracellular ROS generation and oxidative stress. Moreover, silence of VDAC1 increased AMPKα1/2 phosphorylation and suppressed mTOR phosphorylation under BPA exposure. Dorsomorphin, an AMPK inhibitor, partially abolished the effects of VDAC1 gene silencing on BPA-stimulated GC-1 spg cells. In conclusion, inhibition of VDAC1 attenuated the BPA-induced oxidative stress and apoptosis and promoted the cell viability in spermatogonia through modulating AMPK/mTOR signaling pathway.
Bisphenol A (BPA) is one of the highest volume compounds worldwide, and is frequently applied in the production of polycarbonate, epoxy resin and other polymer materials (Lu et al., 2013). BPA is potentially harmful to health via diverse molecular mechanisms (Zhang et al., 2021; Kobayashi et al., 2012; Murata and Kang, 2018; Abraham and Chakraborty, 2020; Minatoya and Kishi, 2021). Scientific evidence has demonstrated that increases the risk for hypertension and cardiovascular disease (Wehbe et al., 2020) and affects glucose metabolism and immune function (Provvisiero et al., 2016; Xu et al., 2016). Current researches are focused on the function of BPA on male fertility. A growing literature proposes the impairment of male reproductive function induced by BPA (Castellini et al., 2020) . In adult rodent models, BPA exposure significantly decreases the sperm counts (Al-Hiyasat et al., 2002) and sperm motility (Tiwari and Vanage, 2013), increases the sperm structural abnormality (Karnam et al., 2015) and sperm DNA damage (Tiwari and Vanage, 2013), and impairs spermatogenesis (Liu et al., 2013). In addition, prenatal BPA exposure resulted in enlarged preputial glands, decreased epididymis, and reduced sperm production efficiency in male mice (vom Saal et al., 1998). Salian et al. (2009a, 2009b, 2011) found that BPA stimulation reduced fertility, daily sperm production, number and motility in offspring of rats. A human study found that BPA exposure might be associated with the decline in total sperm count, semen quality and the increase of sperm DNA damage (Meeker et al., 2010; Chen et al., 2022). Additionally, physiologically detectable concentrations of BPA inhibits the sperm viability, motility and progressive motility of human sperm in vitro (Li et al., 2021). The cell apoptosis of germ cells in the testis was observed from postnatal day (PND) 45 to PND 90 in mice receiving BPA (80 mg/kg/day) from PND 31 to 44 (Li et al., 2009). Administration of BPA at lowest-observed-adverse-effect level (LOAEL) dose significantly reduces the spermatogonia/Sertoli cell ratio in adult male mice (Karmakar et al., 2020). Moreover, BPA reduces the cell count of spermatogonia, spermatocytes and spermatids and increases cell apoptosis of these cells in adult mice testis (Kaur et al., 2018). Studies found that BPA treatment inhibits the activities of antioxidant enzyme and elevates lipid peroxidation levels, ROS production and oxidative stress in testis (De Flora et al., 2011; D'Cruz et al., 2012; Kaur et al., 2018). However, whether BPA induces apoptosis of spermatogonia through ROS-induced oxidative stress are not revealed.
Voltage dependent anion channel 1 (VDAC1) is a pore protein locating on the outer mitochondrial membrane, and serves as a mitochondrial gatekeeper for the transfer of fatty acid ions, reactive oxygen species (ROS), metabolites and proteins between mitochondria and cytoplasm (Shoshan-Barmatz et al., 2018). VDAC1 participates in the process of mitochondria-mediated apoptosis via interacting with anti-apoptotic proteins (Monaco et al., 2015). Considerable evidence supports the function of VDAC1, which is ascribed to oxidative stress and apoptotic properties. VDAC1 inhibitor 4,4’-diisothiocyanostilbene-2,2’-disulfonic acid (DIDS) prevents 5-aminolevulinic acid-induced oxidative stress and apoptosis in THP-1 macrophage (Chen et al., 2014). Overexpression of VDAC1 enhanced oxidative stress-induced apoptosis in cerebellar granule neurons (Liao et al., 2015). Amir and colleagues conducted a clinical trial and found that the mRNA levels of Bax and VDAC1 were significantly elevated in spermatozoa from oligozoospermia patients compared to that in the fertile donors (Amir et al., 2016). Deletion of cystic fibrosis transmembrane conductance regulator (CFTR), an important regulator of male fertility, slightly increased VDAC1 expression in mice testis. The authors proposed that the increased VDAC1 expression and reduced the interactions of Grp78 and VDAC1 might contribute to altered energy metabolism and ROS production in germ cells (Chen et al., 2012; Yan et al., 2016). Additionalyy, a classical traditional Chinese medicine Wuzi-Yanzong-Wan partially reversed the atractyloside-increased apoptosis and VDAC1 expression in mouse spermatocyte GC-2 cells (Wu et al., 2022a). Therefore, in this study, we investigated whether VDAC1 is involved in BPA-induced the apoptosis of spermatogonia.
AMPK/mTOR signaling pathway is a classical signaling pathway for modulating cell apoptosis. AMPK is a critical regulator of cell growth, autophagy and energy metabolism (Feng et al., 2019; Herzig and Shaw, 2018). MTOR is a serine/threo-nine kinase and acts as a downstream effector of AMPK (Wang and Hu, 2021). The AMPK/mTOR signaling pathway has been reported to be involved in spermatogenesis-associated cell apoptosis (Duan et al., 2016) and spermatozoa autophagy (Li et al., 2017). Inhibition of AMPK/mTOR signaling pathway promotes LPS-induced cell death in rat leydig cells (Li et al., 2019). In addition, BPA exposure induced the cell apoptosis and inhibited the cell viability and AMPK/mTOR signaling pathway in macrophage RAW264.7 cells (Wu et al., 2022b). Therefore, whether VDAC1 regulates BPA-induced apoptosis of spermatogonia through modulating AMPK/mTOR signaling pathway is worthy of investigation.
Mouse spermatogonia cell line GC-1 spg cells were purchased from iCell Bioscience Inc. (Shanghai, China) and were cultured in DMEM medium supplemented with 10% fetal bovine serum (Procell Life Science & Technology, Wuhan, China) at 37°C in an incubator with 5% CO2. GC-1 spg cells were treated with various concentrations of BPA (0, 20, 40 and 80 μM) (Macklin, Shanghai, China) or 80 μM alone for 48 hr. The cell morphology of GC-1 spg cells 48 hr after BPA exposure at various concentrations was captured using a microscope. To investigative the role of VDAC1 in the BPA-treated cells, the expression of VDAC1 was silenced by lentivirus-mediated short hairpin RNAs (shRNAs) targeting VDAC1 (Lv-shVDAC1-1, Lv-shVDAC1-2 and Lv-shVDAC1-3). The target sequences for the VDAC1 were as follows: 5’-GGATACACTCAGACTCTAAAG-3’ for Lv-shVDAC1-1, and 5’- GGTTTAGGATACACTCAGACT-3’ for Lv-shVDAC1-2, 5’-GGACAGCAGGAAACAGTAACA-3’ for Lv-shVDAC1-3. The sequence of negative control was 5’-TTCTCCGAACGTGTCACGT-3’. GC-1 spg cells were respectively infected with Lv-shVDAC1-1, Lv-shVDAC1-2, Lv-shVDAC1-3 or lentivirus containing sequence of negative control (Lv-Ctrl) and cultured for 72 hr. Subsequently, the non-infected and infected cells were subjected to BPA exposure (80 μM). In other experiments, the Lv-shVDAC1-infected and Lv-Ctrl-infected cells were exposed to BPA in the absence or presence of an AMPK inhibitor Dorsomorphin (5 μM, Aladdin, Shanghai, China) for 48 hr. Then the further experiments were carried out.
Cell Counting Kit-8 (CCK-8) AssayGC-1 spg cells (4000 per well) were seeded into 96-well plates and cultured in an incubator at 37°C and 5% CO2. After lentivirus infection or appropriate BPA treatment, CCK-8 reagent (10 μL, KeyGen Biotech, Nanjing, China) was added to each well and incubated for 2 hr. The absorbance at 450 nm was examined by a microplate reader (Biotek, Winooski, VT, USA).
Real-time PCRTotal RNA from cells was extracted with TRIpure lysis Buffer (BioTeke Corporation, Beijing, China). mRNA was reversely transcribed to complementary DNA (cDNA) with BeyoRT™ II M-MLV reverse transcriptase (Beyotime Institute of Biotechnology, Shanghai, China). Real-time PCR was performed on ExicyclerTM 96 real-time quantitative thermal block (Bioneer Corporation, Daejeon, Korea) using cDNA, primers (GenScript Corporation, Nanjing, China), SYBR GREEN (Solarbio Science & Technology, Beijing, China) and PCR MasterMix (Solarbio Science & Technology). The expression of GAPDH was used as internal control. The primers purchased were as follows: VDAC1 (Mus musculus) Forward: 5‘-CCGCCAGGGATGTCTTCA-3’, Reverse: 5’-TTTCCAGGCTGCCGTTCA-3’. The 2−ΔΔCt method was used to calculate the relative expression.
Western blotThe cells were lysed in RIPA buffer (Solarbio Science & Technology) and the protein concentration was quantified by BCA protein assay kit (Solarbio Science & Technology). Protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to PVDF membranes (Millipore, Bedford, MA, USA). These membranes were blocked with 5% nonfat milk (Sangon Biotech, Shanghai, China) or 5% BSA and subsequently incubated with the primary antibodies overnight at 4°C. The primary antibodies used in this study were: VDAC1 antibody (1: 500, A19707, ABclonal Biotechnology, Wuhan, China), p-AMPKα1/2 antibody (1: 3000, ab133448, Abcam, Cambridge, UK), AMPKα1/2 antibody (1: 400, AF6423, Affinity Biosciences, Changzhou, China), Bax antibody (1: 1000, A19684, ABclonal, Wuhan, China), Bcl-2 antibody (1: 500, A19693, ABclonal), p-mTOR antibody (1: 1000, AF3308, Affinity Biosciences), mTOR antibody (1: 500, AF6308, Affinity Biosciences), cleaved-Caspase 3 antibody (1: 500, AF7022, Affinity Biosciences), cleaved-Caspase 9 antibody (1: 1000, #9507, Santa Cruz Biotechnology, Dallas, TX, USA), GAPDH (1: 10000, T0004, Affinity Biosciences). These membranes then were incubated with secondary antibodies including goat anti-rabbit IgG/HRP antibody (1: 3000, SE134, Solarbio Science & Technology) or goat anti-mouse IgG/HRP (1: 3000, SE131, Solarbio Science & Technology). The protein bands on membranes were observed using enhanced chemiluminescence (ECL) Plus reagents (Solarbio Science & Technology). The signals were quantified with a gel image analysis system. GAPDH was used as internal control.
Apoptosis analysisCell apoptosis of GC-1 spg cells was evaluated using the Annexin V-FITC/Propidium Iodide (PI) apoptosis detection kit (KeyGen Biotech) and TUNEL staining assay according to the manufacturer’s instructions. Briefly, cells for Annexin V-FITC/PI staining were collected and incubated with 5 μL Annexin V-FITC and 5 μL PI in 500 μL binding buffer for 15 min in darkness. The stained cells were analyzed by a NovoCyte flow cytometry (ACEA Biosciences, San Diego, CA, USA). The cells for TUNEL staining were permeabilized with 0.1% Triton X-100 (Beyotime Institute of Biotechnology) and then stained with TUNEL staining solution (Roche, Basel, Switzerland) for 60 min. The cells were stained with 4',6-diamidino-2-phenylindole (DAPI, Aladdin) and observed using a fluorescence microscope.
Superoxide Dismutase (SOD), Lactate Dehydrogenase (LDH) and Malondialdehyde (MDA) detection assaysCells were broken using ultrasonication in ice and centrifuged to collect supernatant. The activity of SOD and the content of MDA were determined using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. The cell supernatant was collected and centrifuged, and the activity of lactate dehydrogenase (LDH) was detected by LDH detection kit (Wanleibio, Shenyang, China) according to the manufacturer’s procedure.
Reactive oxygen species (ROS) detection assayIntracellular ROS was determined with reactive oxygen species assay kit (KeyGEN Biotech) according to the manufacturer’s instructions. Cell suspension was washed with PBS, and incubated with 1 mL DCFH-DA for 20 min. The cells were washed with PBS three times and the intracellular ROS level was detected by a flow cytometry.
Statistical AnalysisData was presented as means ± standard deviation (SD) and analyzed by GraphPad Prism 8 software. The differences between Control and BPA groups were analyzed using the student’s t-test. One-way or two-way analysis of variance (ANOVA) was used to perform comparisons among other multiple groups. Differences were considered statistically significant at P < 0.05.
To investigate the effects of BPA on the cell viability of spermatogonia, GC-1 spg cells were treated with BPA with various concentrations (0, 20, 40 and 80 μM) for 48 hr. The cell viability of cells was significantly reduced at BPA high concentration (80 μM) (Fig. 1A). In addition, the decreased cell number of GC-1 spg cells was observed in cells treated with 80 μM BPA (Fig. 1B). This indicated that BPA was toxic to GC-1 spg cells at a concentration 80 μM. Thus 80 μM BPA was used for further experiment. To verify whether BPA induces apoptosis and oxidative stress in GC-1 spg cells, we examined apoptotic cells and intracellular ROS level after BPA exposure. The results revealed that BPA exposure significantly increased cell apoptosis that is determined by Annexin V-PI staining and TUNEL staining (Fig. 1C, D). BPA exposure also significantly enhanced ROS level (Fig. 1E). This indicates that BPA decreases cell viability and increases cell apoptosis and intracellular ROS level in GC-1 spg cells.
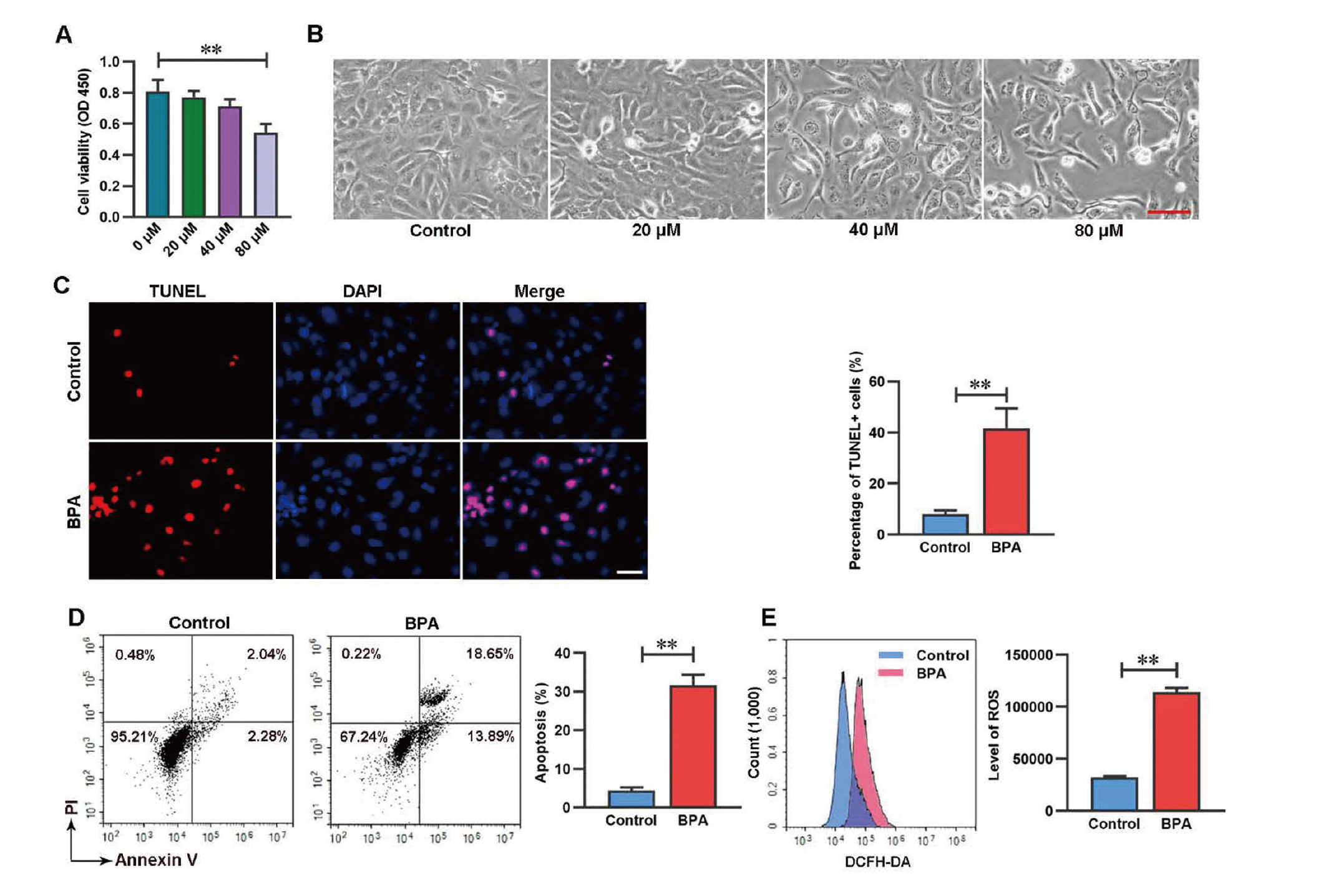
BPA suppressed the cell viability and induced cell apoptosis and ROS generation of GC-1 spg cells. (A) Cell viability of GC-1 spg cells exposed to BPA (0, 20, 40 and 80 μM) for 48 hr. (B) Representative images were taken 48 hr after BPA exposure to show the cell morphology of GC-1 spg cells (scale bar = 100 μm). (C) The apoptotic cells were observed by TUNEL staining (scale bar = 50 μm). (D) The percentage of BPA-treated apoptotic cells among the total number of cells was determined by Annexin V-FITC/PI staining and flow cytometry. (E) Intracellular ROS levels in GC-1 spg cells in the control and BPA treatment groups. Data was represented as the mean ± SD. n = 3. **P < 0.01.
To explore whether VDAC1 plays an important role in BPA-induced cell apoptosis, the mRNA and protein levels of VDAC1 were detected in BPA-treated cells. The results showed that BPA increased the mRNA and protein levels of VDAC1 (Fig. 2A, B). Additionally, the protein expression of AMPK and mTOR was examined. As indicated in Fig. 2C and D, BPA treatment remarkably inhibited the phosphorylated levels of AMPKalpha1/2 (p-AMPKα1/2) and elevated the phosphorylated levels of mTOR (p-mTOR) in GC-1 spg cells. These results imply that BPA exposure elevates VDAC1 expression and inhibits the AMPK/mTOR signaling pathway in GC-1 spg cells.
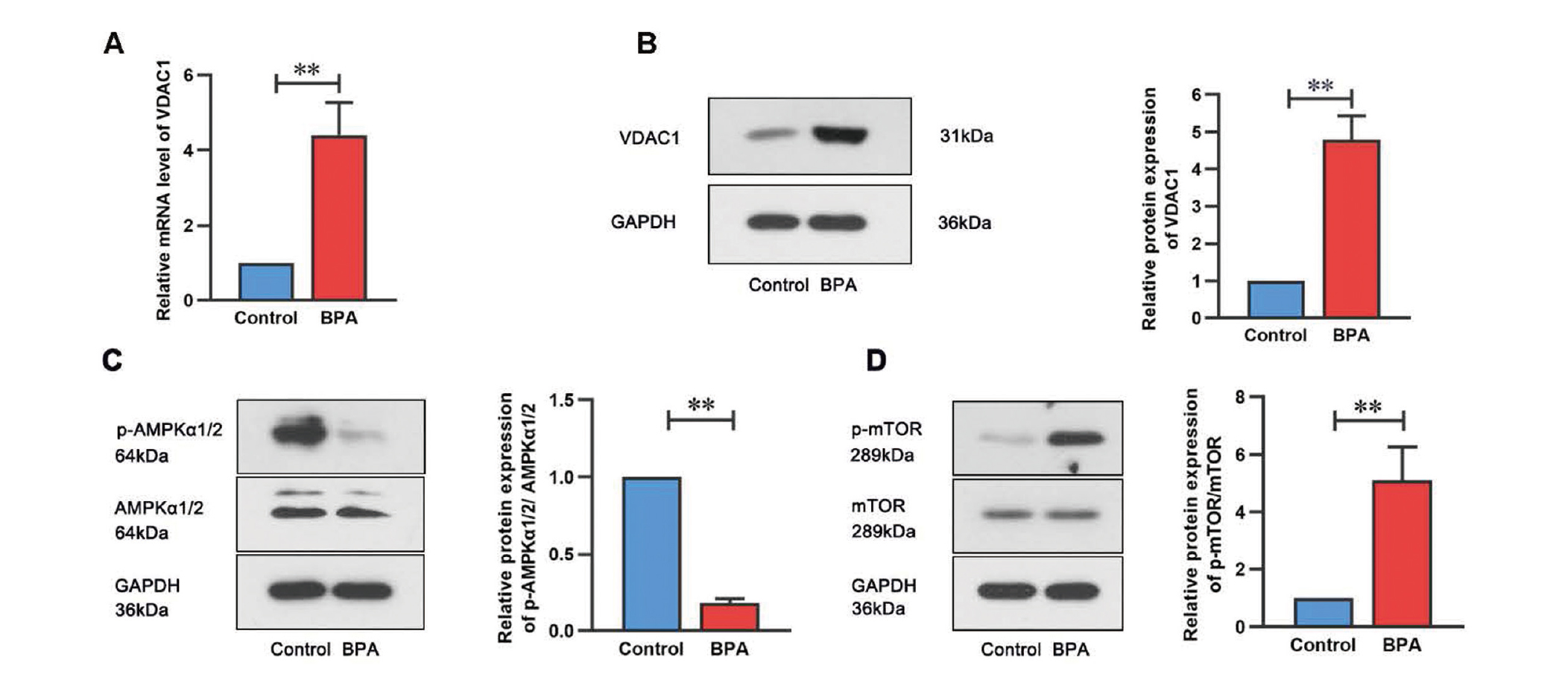
BPA treatment affected VDAC1 expression and AMPK/mTOR pathway in GC-1 spg cells. (A, B) GC-1 spg cells were treated with 80 μM BPA for 48 hr, the mRNA level and protein level of VDAC1 were detected by real-time PCR and western blot. (C, D) The protein levels of AMPKα1/2, p-AMPKα1/2, mTOR and p-mTOR were evaluated by western blot. Data was represented as the mean ± SD. n = 3. **P < 0.01.
To investigative the role of VDAC1 in apoptosis and oxidative stress of GC-1 spg cells, Lv-shVDAC1 was infected into GC-1 spg cells to downregulate VDAC1 expression. The results in Fig. 3A and B showed that Lv-shVDAC1-1, Lv-shVDAC1-2 and Lv-shVDAC1-3 infection effectively suppressed VDAC1 expression. Lv-shVDAC1-3-infected cells had a higher effectively to silence VDAC1. Therefore, Lv-shVDAC1-3 was used for further study and termed it as Lv-shVDAC1. We found that knockdown of VDAC1 increased the cell viability compared to that in Lv-shCtrl group under BPA exposure (Fig. 3C). TUENL staining and flow cytometric analysis showed that the percentages of apoptotic cells were dramatically elevated in BPA-treated GC-1 spg cells, whereas VDAC1 gene silencing significantly reduced apoptosis induced by BPA in GC-1 spg cells (Fig. 3D, E). The levels of pro-apoptotic protein Bax, cleaved-Caspase 3, and cleaved-Caspase 9 were significantly elevated while anti-apoptotic protein Bcl-2 was remarkably suppressed after BPA exposure. However, the expression levels of these proteins were partially reversed by VDAC1 knockdown in BPA-stimulated cells (Fig. 3F). We further explored the role of VDAC1 in oxidative stress by detecting ROS level and antioxidant enzyme content and activities. The increase of ROS production, MDA content, LDH activity in BPA-stimulated GC-1 spg cells was markedly restrained by VDAC1 knockdown. By contrast, knockdown of VDAC1 increased the BPA-reduced SOD activity (Fig. 3G–J). These data indicate that knockdown of VDAC1 contributes to prevent cell apoptosis and ameliorates oxidative stress in GC-1 spg cells to BPA exposure.
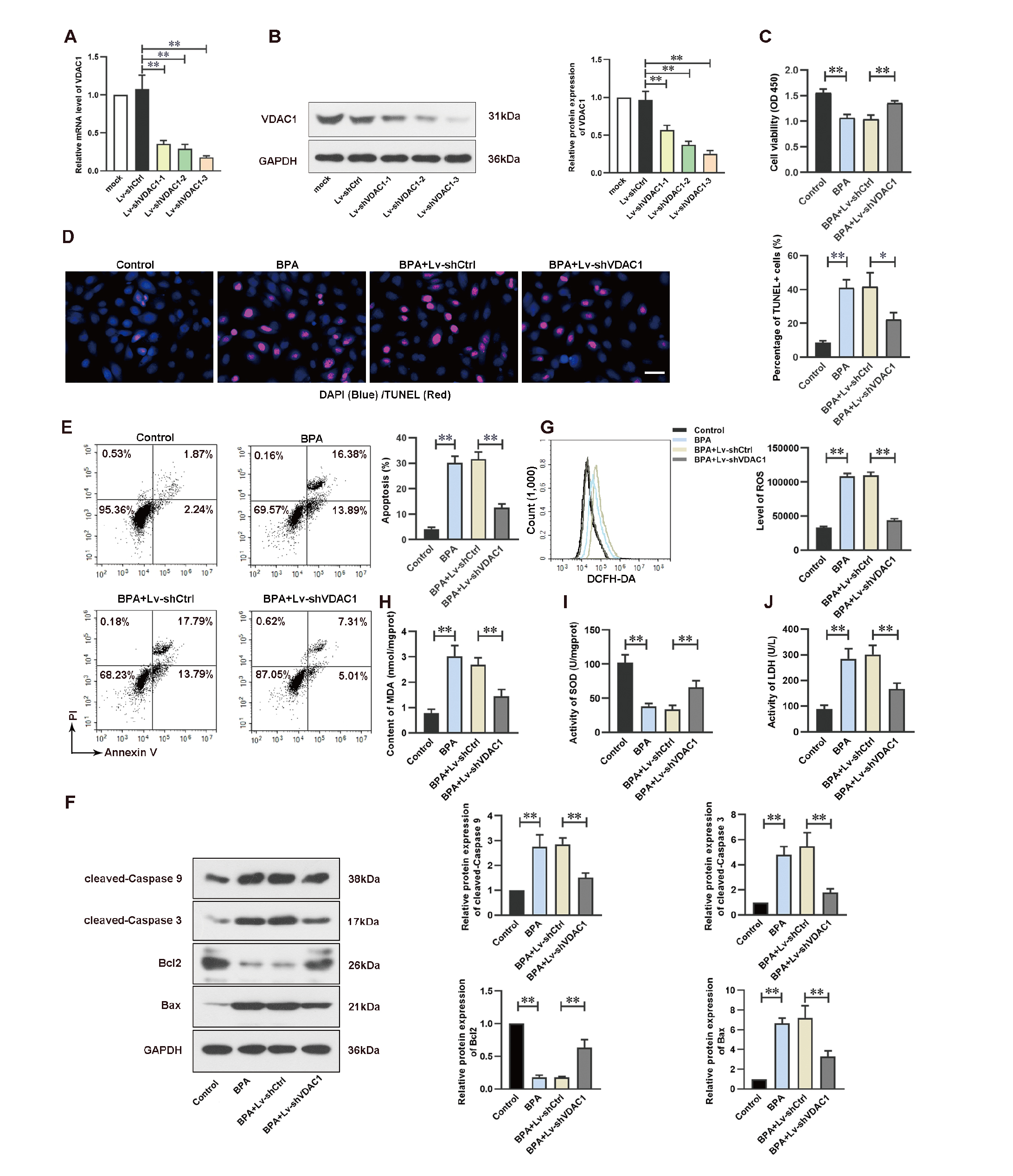
VDAC1 knockdown attenuated BPA-induced oxidative stress and apoptosis in GC-1 spg cells. GC-1 spg cell were infected with lentivirus-mediated shRNAs targeting VDAC1 (Lv-shVDAC1-1, Lv-shVDAC1-2 and Lv-shVDAC1-3) or negative control (Lv-shCtrl) and then the infected cells were exposed to BPA at a dose of 80 μM for 48 hr. (A, B) The mRNA and protein levels of VDAC1 in infected GC-1 spg cells were determined by real-time PCR and western blot. (C) GC-1 spg cells were infected with Lv-shVDAC1 (Lv-shVDAC1-3) or Lv-shCtrl and then treated with BPA for 48 hr. Cell viability of GC-1 spg cells was deteced by CCK-8 assay. (D, E) The apoptotic cells were observed by TUNEL staining (scale bar = 50 μm) and Annexin V-FITC/PI staining in VDAC1-silenced cells treated with BPA for 48 hr. (F) The protein levels of cleaved-Caspase 9, cleaved-Caspase 3, Bcl2 and Bax in the GC-1 spg cells were measured by western blot. Intracellular ROS production (G), MDA level (H), SOD activity (I) and LDH activity (J) in the GC-1 spg cells. Data was represented as the mean ± SD. n = 3. **P < 0.01, *P < 0.05.
The AMPK/mTOR signaling pathway is participated in apoptosis (Xie et al., 2022) and cell proliferation (Chen et al., 2021). The effect of VDAC1 on AMPK/mTOR signaling pathway was assessed. As shown in Fig. 4A and B, silence of VDAC1 promoted activation of AMPK/mTOR signaling pathway by enhancing p-AMPKα1/2 expression and decreasing p-mTOR expression.

Knockdown of VDAC1 activated AMPK/mTOR signaling pathway in BPA-treated GC-1 spg cells. (A, B) Expression of p-AMPKα1/2, AMPKα1/2, p-mTOR and mTOR in the GC-1 spg cells infected with Lv-shVDAC1 or Lv-shCtrl was analyzed by western blot. Data was represented as the mean ± SD. n = 3. **P < 0.01.
To further verify whether VDAC1 affects BPA-treated cell injury and apoptosis through AMPK/mTOR signaling pathway, Lv-shVDAC1-infected GC-1 spg cells were treated with BPA in the absence or presence of an AMPK inhibitor Dorsomorphin. The results showed that Dorsomorphin treatment in Lv-shVDAC1-infected cells downregulated the p-AMPKα1/2 level and upregulated the p-mTOR level under BPA exposure (Fig. 5A, B). Inactivation of AMPK reduced the cell viability in Lv-shVDAC1-infected cells (Fig. 5C). In addition, Dorsomorphin reversed the inhibition of cell apoptosis by VDAC1 silence after BPA exposure (Fig. 5D–F). Dorsomorphin also increased the LDH activity and ROS level in Lv-shVDAC1-infected cells (Fig. 5G, H). Collectively, these current results showed that inactivation of AMPK partially abolished the effects of VDAC1 silence on BPA-stimulated GC-1 spg cells. This indicates that VDAC1 promotes BPA-treated cell injury and apoptosis through AMPK/mTOR signaling pathway.
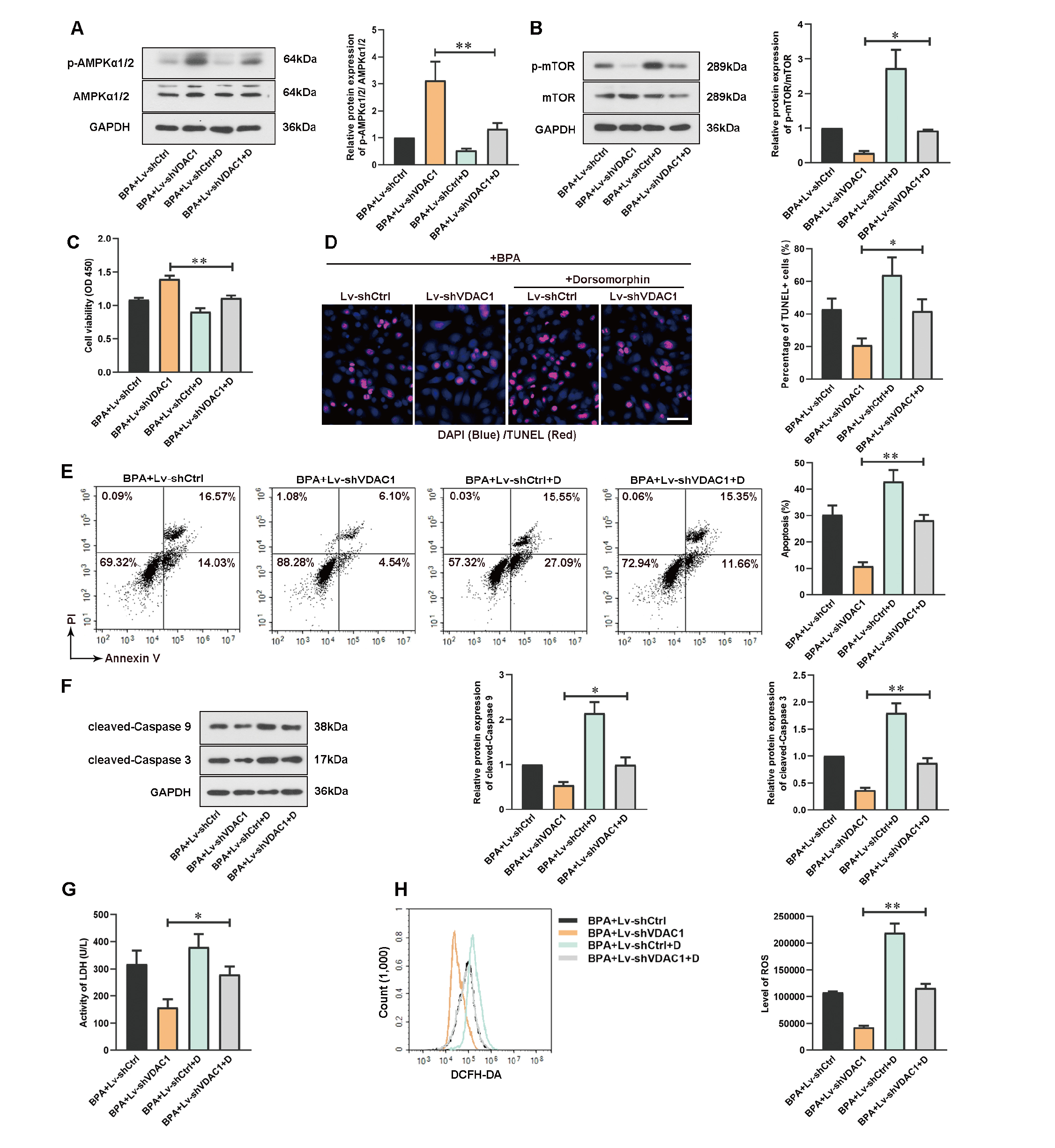
VDAC1 acted its role in BPA-treated GC-1 spg cells by mediating AMPK/mTOR signaling pathway. GC-1 spg cells were infected with Lv-shVDAC1 or Lv-shCtrl and then treated with BPA and with/without 5 μM Dorsomorphin (D) for 48 hr. (A, B) Western blot was applied to determine protein expression levels of AMPKα1/2, p-AMPKα1/2, mTOR and p-mTOR in BPA-treated GC-1 spg cells. (C) Cell viability of GC-1 spg cells was detected by CCK-8 assay. (D) The apoptotic cells were observed by TUNEL staining (scale bar = 50 μm). (E) Flow cytometry analysis was performed to assess the apoptosis in GC-1 spg cells using with double staining of Annexin V-FITC and PI. (F) The protein levels of cleaved-Caspase 9 and cleaved-Caspase 3 in GC-1 spg cells were tested by western blot. (G, H) LDH activity was measured using commercial kit and ROS production was detected by flow cytometry. Data was represented as the mean ± SD. n = 3. *P < 0.05, **P < 0.01.
BPA is an environmental endocrine disruptor that has been widely studied because of its prevalence in life (Lee et al., 2021). Reliable evidence supports that BPA has the potential to induce male reproductive toxicity in animals and humans. Karnam et al. (2015) reported that the administration of BPA for 28 days significantly decreased the total sperm count and sperm motility percentage and increased the sperm abnormality percentage using 6 weeks old male Wistar rats. Liu et al. (2021) found that administration of BPA to adult male mice for two months impaired cell proliferation of germ cells including spermatogonial cells in vivo and BPA exposure inhibited GC-1 spg cells in vitro. In addition, treatment of BPA inhibited cell viability and induced cell apoptosis and ROS accumulation of mouse spermatocyte GC-2 cells in vitro and administration of BPA promoted the apoptosis of spermatogenetic cells in mice testes (Yin et al., 2017). In this study, we tried to reveal the effects and potential molecular mechanisms of BPA on spermatogonia. Our results demonstrated that BPA exhibited a toxic effect on spermatogonia by inducing ROS accumulation and suppressing the AMPK/mTOR signaling pathway, which ultimately led to cell apoptosis and oxidative stress.
Oxidative stress is the unbalanced status between oxidation and anti-oxidation and often takes part in the development of diseases (Sies, 2015; Forman and Zhang, 2021). Under healthy condition, ROS produced by mitochondria are essential for normal cellular signaling and regulating cellular biological processes. However, ROS level is significantly elevated when the cell is in a state of oxidative stress (Singh et al., 2019). Previous studies have proved that BPA disrupts redox homeostasis, induces ROS accumulation and oxidative stress (Zhang et al., 2021; Zhao et al., 2019). BPA treatment to rat leads to thyroid oxidative damage associated by overproduction of MDA, elevated inducible nitric oxide synthase (iNOS) expression, and reduced SOD activity (Mohammed et al., 2020). Consistently, our results clearly demonstrated that the BPA exposure induced ROS overgeneration and decreased antioxidant enzyme activity, which resulted in the imbalance between oxidant and antioxidant actions in spermatogonia and induced oxidative stress.
VDAC1 is highly expressed in the gonocytes of male rat PND3, the spermatogonia of PND8 and its expression is decreased in adult germ cells of PND60. However, the expressions of VDAC2 and VDAC3 are opposite to the expression of VDAC1 (Culty et al., 2015). Sampson et al. (2001) found that VDAC1 and VDAC3 were expressed in mitochondria of testicular cells in adult mice. VDAC3-deficient male mice are infertile because of the reduced sperm motility and increased sperm structural abnormality (Sampson et al., 2001). These studies suggest that VDACs have different functions in germ cells and the low expression of VDAC1 in adult germ cells including spermatogonia might be beneficial to spermatogenesis. Interestingly, we found that VDAC1 was dramatically up-regulated in BPA-stimulated GC-1 spg cells. Therefore, we speculated VDAC1 might be associated with BPA-induced spermatogonium damage. The VDAC1 is in the outer membrane of mitochondria and plays an important role in cell apoptosis. For instance, overexpression of VDAC1 aggravates the myocardial ischemia/reperfusion injury by promoting mitochondria-mediated apoptosis of cardiomyocytes (Lin et al., 2018). Additionally, VDAC1 inhibitor DIDS inhibits the 5-aminolevulinic acid-induced cell apoptosis of THP-1 macrophages by restoring the mitochondrial membrane disruption and reducing the mitochondria-mediated apoptosis (Chen et al., 2014). VDAC1 small interfering RNA ameliorates mitochondrion-mediated renal cell apoptosis and is participated in the progression of acute kidney injury (Li et al., 2022). Moreover, VDAC1 plays a vital role in mediating cell apoptosis and is also the target of many pro-apoptotic compounds (Tewari et al., 2015; Rimmerman et al., 2013). Our study demonstrated that VDAC1 knockdown weakened BPA-exposed cell apoptosis and attenuated BPA-inhibited cell viability of spermatogonia. A study found that VDAC1 silencing protectes cardiomyocyte from cytotoxic effect of H2O2 as evidenced by reducing in LDH activity and dead cell number (Jiang et al., 2018). Knockdown of VDAC1 inhibits cell death on in vitro model of oxidative stress (de Sousa et al., 2022). Moreover, VDAC1 inhibitor suppresses the generation of intracellular ROS in macrophages (Chen et al., 2014). Consistent with these researches, we observed the knockdown of VDAC1 suppressed LDH activity and ROS generation in BPA-exposed spermatogonia. This implies that VDAC1 might take a dominating position in mitochondria-mediated apoptosis and regulating ROS generation.
The AMPK/mTOR signaling pathway is important for cell apoptosis (Kazyken et al., 2019; Wang and Hu, 2021). For instance, Quercetin inhibits oxidative stress-induced apoptosis of chondrocytes by promoting AMPK phosphorylation (Feng et al., 2019). Overexpression of aprosin induces apoptosis of β‐cells through inhibiting AMPK/mTOR signaling pathway (Wang and Hu, 2021). More importantly, BPA also could induce cell apoptosis and inflammatory and inactivate AMPK/mTOR signaling pathway (Wu et al., 2022b). Emerging evidence displayed that the activation of AMPK/mTOR signaling pathway was related to reproductive cell injury. HT-2 toxin increased the expression levels of Bax and caspase-9 in spermatogonial stem cells through regulating AMPK/mTOR signaling pathway (Pang et al., 2021). Adrenomedullin inhibited LPS-induced cell death of leydig cells through activating AMPK/mTOR signaling pathway (Li et al., 2019). In this study, we found that the phosphorylation of AMPKα1/2 was restrained while mTOR phosphorylation was elevated in BPA-exposed GC-1 spg cells. We further demonstrated that AMPK/mTOR signaling pathway was activated in VDAC1-silenced cells, and knockdown of VDAC1 attenuated apoptosis rate and ROS level. To further investigate the molecular mechanism of VDAC1 in regulating oxidative stress and subsequent apoptosis events, Dorsomorphin was used to inactivate AMPK. Our results showed that Dorsomorphin treatment weakened the effects of VDAC1 silence on cell apoptosis, cell viability and ROS production in BPA-treated GC-1 spg cells. These data clearly imply that VDAC1 promotes the cell apoptosis and oxidative stress of spermatogonia to BPA exposure by partially modulating AMPK/mTOR signaling pathway.
In conclusion, these findings demonstrated that the expression of VDAC1 is up-regulated in BPA-treated GC-1 spg cells. Knockdown of VDAC1 prevented BPA-induced spermatogonia from apoptosis and oxidative stress through regulating AMPK/mTOR signaling pathway. The current study provide a regulatory mechanism of BPA-induced male reproductive toxicity.
Conflict of interestThe authors declare that there is no conflict of interest.